HIV testing should be administered with pre and post-test counselling and informed oral consent.
- HIV antibody rapid tests will be conducted using the blood specimen collected. Seven millilitres (7 mls) of venous blood will be drawn from the client, or blood may be obtained by finger prick for immediate, onsite testing. For venous samples, the specimen may be transported to testing sites within the parish or testing may be conducted on site.
- The Laboratory Technical Assistant (LTA) or designated individual will carry out the test.
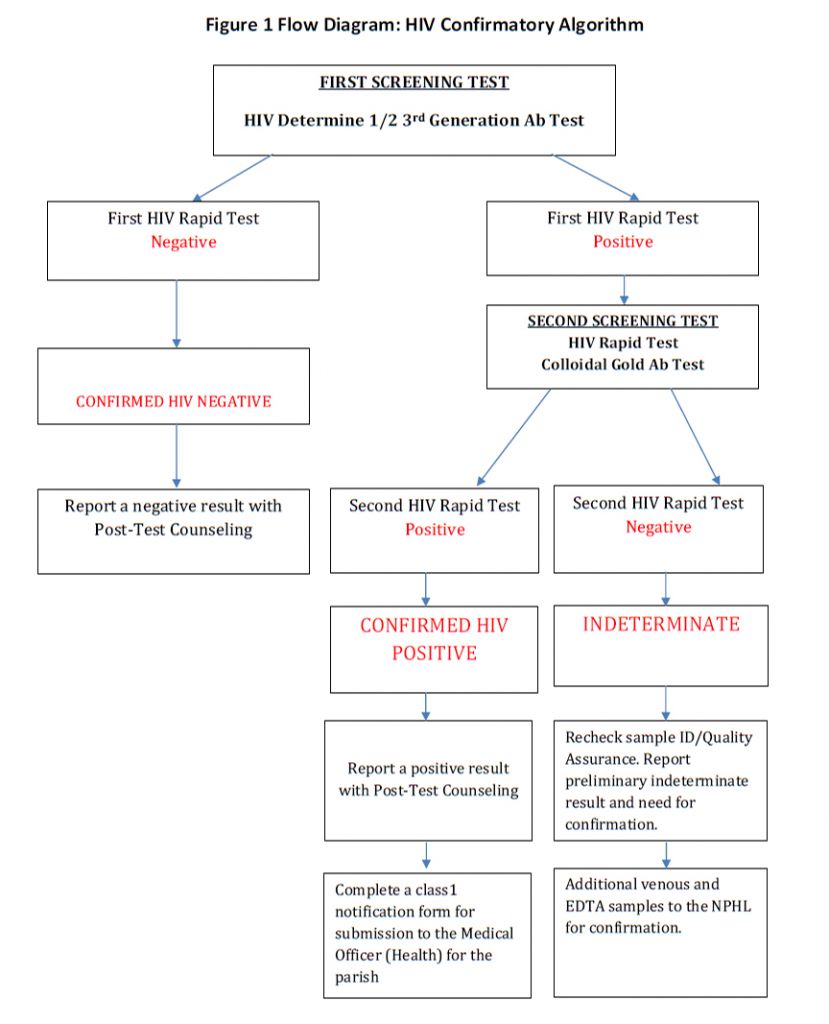
- The Determine HIV 1/2-test kits will be utilized as outlined in the attached algorithm as the initial screen (sensitivity- 98.22% and specificity- 99.79 %) (Figure 1)
- A Colloidal Gold HIV 1/2test will be performed on all Determine Positives
- A positive Determine test and a positive Colloidal Gold test will be a confirmed positive HIV test and should be communicated to the patient and appropriate counselling/interventions commenced.
- Additional samples from clients with indeterminate results and from women testing positive for the first time during pregnancy are to be taken and sent to the NPHL for confirmation (both a clotted sample and an EDTA sample are required).
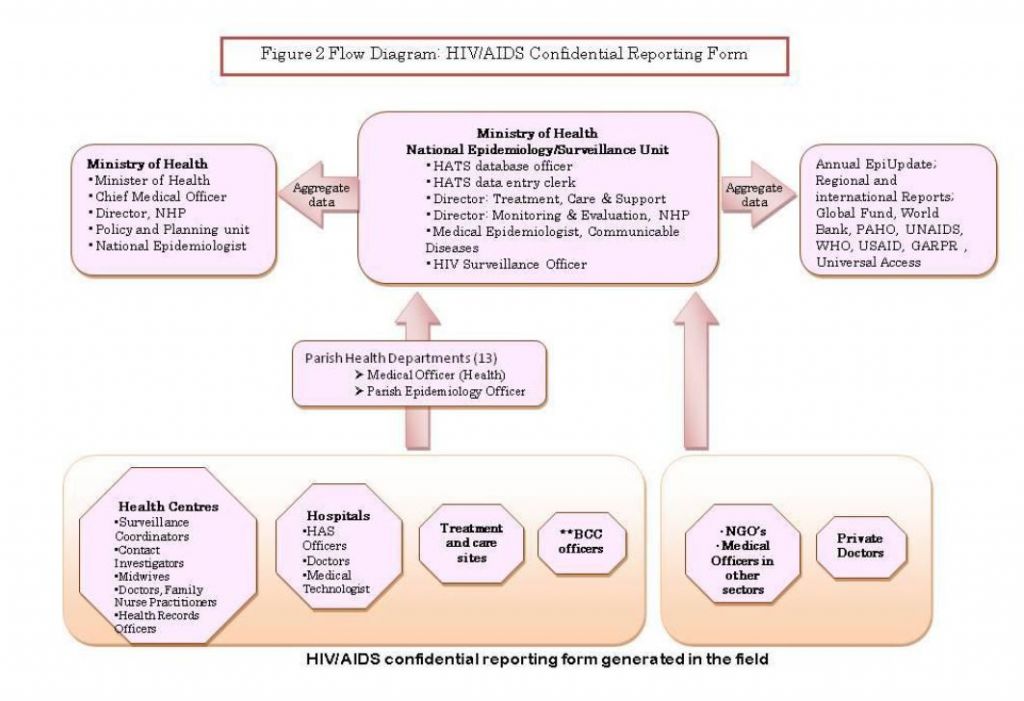
All test results will be made available to the referring institution and to the patient within one week of testing. Priority will be given to HIV positive results which should be sent immediately to the institution for early identification, follow up and treatment as per guidelines. The positive results should be sent directly to PHN, CI or physician who ordered the test or is managing the patient in each parish to ensure that confidentiality is maintained. A class1 notification form and a HIV/ADIS Confidential Reporting form are to be submitted to the Ministry of Health (Figure 2).